Research projects
Create2Solve
 The nominal concentration, i.e. the theoretical concentration based on amount of test chemical added to culture medium, is generally used to express concentration-effect relationships in in vitro toxicity tests. However, for instable, volatile, lipophilic and highly plasma protein bound chemicals, the nominal concentration does not represent the concentration responsible for the observed effects at the target site in cells. In this project, we develop tools that control for the degradation, evaporation and binding of chemicals to the in vitro system setup of these chemicals. These tools include exposing cells in sealed glass vials and microtiter plates dosed through polymers loaded with test chemicals (i.e. partition-controlled dosing). A decision tree will be evaluated to allow researchers to determine when to use these dosing tools based on the properties of the test chemical and in vitro test system. The project is funded by ZonMW and is perfomed in collaboration with Vivaltes, Toxys and the Dutch Institute for Public Health and the Environment (RIVM).
The nominal concentration, i.e. the theoretical concentration based on amount of test chemical added to culture medium, is generally used to express concentration-effect relationships in in vitro toxicity tests. However, for instable, volatile, lipophilic and highly plasma protein bound chemicals, the nominal concentration does not represent the concentration responsible for the observed effects at the target site in cells. In this project, we develop tools that control for the degradation, evaporation and binding of chemicals to the in vitro system setup of these chemicals. These tools include exposing cells in sealed glass vials and microtiter plates dosed through polymers loaded with test chemicals (i.e. partition-controlled dosing). A decision tree will be evaluated to allow researchers to determine when to use these dosing tools based on the properties of the test chemical and in vitro test system. The project is funded by ZonMW and is perfomed in collaboration with Vivaltes, Toxys and the Dutch Institute for Public Health and the Environment (RIVM).InnoSysTox Risk-IT project
 The aim of the Innosystox RISK-IT project is to provide a proof-of-concept for integrating adverse outcome pathway (AOP)-based in vitro assays and quantitative in vitro-in vivo extrapolation (QIVIVE) modelling into a tiered testing strategy for repeat-dose nephrotoxicity testing.
The aim of the Innosystox RISK-IT project is to provide a proof-of-concept for integrating adverse outcome pathway (AOP)-based in vitro assays and quantitative in vitro-in vivo extrapolation (QIVIVE) modelling into a tiered testing strategy for repeat-dose nephrotoxicity testing.
The predictivity of human nephrotoxicity by animal tests is low owing to interspecies differences in active transporters and metabolizing enzymes. Thus the need for alternative, human-relevant in vitro nephrotoxicity assays is pressing. We are developing AOPs for nephrotoxicity via lysosomal overload and mitochondrial DNA-interaction by exposing human RPTEC/TERT1 and rat NRK-52E cells to, amongst others, polymyxin B, colistin, cidofovir and tenofovir. A selection of in vitro biomarkers is measured over time using high-content imaging, qPCR and metabolomics. To quantify the AOPs, changes in in vitro biomarkers over time are compared to analytically determined cell-associated concentrations of chemicals perturbing the AOPs. We construct physiologically based kinetic (PBK) models to estimate concentrations of the nephrotoxicants in proximal tubule cells in rats and humans. This allows us to compare in vitro effect concentrations with toxic doses in rats and humans. The project is funded by ZonMW and BMBF. For more information, see http://www.toxikologie.uni-wuerzburg.de/en/coordinated-joint-research-projects/risk-it-zonmwbmbf/
Cosmetics Europe LRSS Ontology project
 The aim of the Cosmetics Europe LRSS Ontology project is to develop a repeat dose toxicity ontology model for chemical safety assessment purposes with drug-induced liver injury as a case study.
The aim of the Cosmetics Europe LRSS Ontology project is to develop a repeat dose toxicity ontology model for chemical safety assessment purposes with drug-induced liver injury as a case study.
The main objective of Cosmetics Europe’s LRSS for 2016-2020 is the development of a strategy to determine safety of cosmetic ingredients and products without the use of animal experimentation. Following a series of LRSS expert workshops in 2016 and 2017, it became clear that there is a specific need to develop a systematic representation of knowledge from non-animal test data sources about systemic repeat dose toxicity. An ontology model provides such necessary structure for using this data. An ontology is a knowledge structure of toxicokinetic and toxicodynamic events associated with disease, which can be populated with data from in silico and in vitro sources. Hence, the aim of the Ontology project is to show that a repeat dose toxicity ontology can be used to structure in silico and in vitro data to effectively assess the risk of chemicals to induce liver necrosis, hypertrophy, steatosis and cholestasis after repeated exposure. The liver is the most frequently targeted organs by chemicals, including cosmetics. Using the human hepatoma cell line, HepaRG, high content imaging, transcriptomics, read-across and generic physiologically based pharmacokinetic models, the four pillars of ontology (assessing chemical and structural features of liver toxicants, in vivo and in vitro toxicokinetics of liver toxicants, molecular mechanisms underlying chemical-triggered liver toxicity, in vivo toxicity data mining from literature) are addressed. The project is funded by Cosmetics Europe.
CosEU IV-Kin project
 The aim of the CosEU IV-Kin project is to identify and fill the gaps in modelling the kinetics of cosmetic ingredients in in vitro hepatic clearance assays and in vitro repeat-dose toxicity assays.
The aim of the CosEU IV-Kin project is to identify and fill the gaps in modelling the kinetics of cosmetic ingredients in in vitro hepatic clearance assays and in vitro repeat-dose toxicity assays.
The absorption, distribution, metabolism and excretion of chemicals (ADME) play a central role in quantitative in vitro in vivo extrapolation (QIVIVE) studies, as these processes determine the concentration of toxicants at the target organ where toxic effects are initiated. Although largely ignored, similar kinetic processes determine the target concentration and thus the level of bioactivity of toxicants in in vitro assays. A few models exist relating chemical structure to the extent to which a chemical distributes to medium constituents, well plate plastic, headspace and cells. The aim of the IV-Kin project is to 1. review in vitro kinetics models and analytical measurement techniques to asess in vitro kinetics in the context of cosmetics ingredients, 2. determine medium, plastic and cell-associated concentrations of case study chemicals over time after a single dose and after repeated dosing in in vitro clearance and toxicity assays with hepatocytes, and 3. use the collated data to improve existing in vitro kinetic models to predict in vitro kinetics of cosmetics ingredients. The project is funded by Cosmetics Europe.
EFSA MoHV project
 The aim of the EFSA MoHV project is to develop in vitro test batteries assessing key event perturbations in the steatosis and neurotoxicity adverse outcome pathways (AOP) to screen for the variation in toxic potencies in humans.
The aim of the EFSA MoHV project is to develop in vitro test batteries assessing key event perturbations in the steatosis and neurotoxicity adverse outcome pathways (AOP) to screen for the variation in toxic potencies in humans.
Humans differ in their responses to chemical exposure. Some people experience adverse health effects at lower exposure levels than others. Traditionally, a composite uncertainty factor (10) is applied in non-cancer risk assessment to extrapolate safe levels of exposure for the average individual to all individuals, which allows for interindividual differences in toxicokinetics (TK, 100.5= 3.16) and toxicodynamics (TD, 100.5= 3.16). To move away from default factors to applying chemical specific adjustment factors, the EFSA MoHV project applies quantitative reviews to therapeutic drug databases to characterise human variability in kinetic and dynamic profiles and categorise variability by the metabolic and adverse outcome pathway (AOP) of chemicals. At ATX, we are developing a series of in vitro assays and accompanying TK/TD models to screen for differences in toxic potencies between donors of chemicals perturbing hepatic steatosis and acetylcholinesterase (AChE) inhibition AOPs. The project is funded by the European Food Safety Authority.
The EMERCHE Project

The EMERCHE project aims to implement, using the adverse outcome pathway (AOP) concept, a battery of sensitive and high-throughput bioassays for effect-directed monitoring (EDM) of water quality.
The project is divided into two parts: a screen of ecotoxicological assays is perfomed by a PhD student at WUR and a screen of bioassays for human toxicological risk assessment is performed by a PhD student at IRAS. The selected biological models cover the most critical human and ecological adverse outcomes, taking into account mixture effects of all chemicals (known and unknown). The bioassays describe a relevant toxicological “fingerprint” for ecological and human risk assessment of water bodies. To do so, the project links the protection goals for humans and ecosystems described in (supra)national guidelines and a list of prioritized relevant chemicals with related adverse outcome pathways (AOP). Bioassays will be selected based on key events (KE) that lead to adverse human and ecological outcomes in relevant AOPs. Then, trigger values are derived for each selected bioassay using, among others, toxicokinetic models (e.g. QIVIVE, TK/TD). Afterwards, the EDM tools are validated; firstly with a mesocosm study where selected and known compounds are tested in a mixture, and, secondly, with a water monitoring program to assess the added benefit of the assays and ease of use for water quality assessors. The challenge is to propose specific tools which will be practical and easy-use for the end-users and stakeholders, while being relevant, sensitive and robust. The project is funded by the Dutch NWO Partnership program TTW‐STOWA‐KWR‐TKI Watertechnology.
http://www.cec-partnership.nl/web/index.php/projects/emerche
TTW LIVeCON project
The LIVeCON project’s aim is to fabricate liver constructs for human hepatotoxicity testing using human liver organoids and 3D bioprinting.
Robust and readily available in vitro assays for predicting drug-induced liver injury and for developing personalized drug treatments have yet to be developed. To improve the functionality of hepatocytes in vitro and account for interindividual variation in liver responses to drug treatment, liver organoids derived from adult stem cells are used as they represent a novel donor-specific model. These organoids are hollow spheroid-like structures, able to differentiate towards cholangiocyte-like cells and hepatocyte-like cells. The complex liver physiology and architecture of these organoids are recapitulated by bioprining 3D constructs and subsequently exposing these constructs to drugs in a flow-perfusion 3D tissue bioreactor. The project is funded by NWO TTW and is coordinated by Dr. Bart Spee. It is done in collaboration with IRAS and UMCU.
https://www.nwo.nl/onderzoek-en-resultaten/onderzoeksprojecten/i/33/28933.html
in3 project
 The aim of the in3 project is to derive a strategy to integrate in vitro assays with human induced pluripotent stem cells (hiPSC) and in silico tools for human chemical and nanomaterial (NM) safety assessment.
The aim of the in3 project is to derive a strategy to integrate in vitro assays with human induced pluripotent stem cells (hiPSC) and in silico tools for human chemical and nanomaterial (NM) safety assessment.
In3 is an EU’s Marie Sklodowska-Curie Action – Innovative Training Network (MSCA-ITN) and consists in 15 PhD students stationed throughout Europe and all aiming to reduce, refine and replace animal testing (3R) in the human safety assessment of chemicals and nanomaterial by using human induced Pluripotent Stem Cells (hiPSC). hiPSC are differentiated into brain, lung, liver and kidney cells. The cells, with the same genetic background, are exposed to 10 known neurotoxicants, hepatotoxicants, nephrotoxicants and respiratory toxicants. At ATX, we assess the in vitro distribution of these toxicants in the different in vitro setups used in the project determine if differences in the distribution explains the differences in sensitivity in toxicity readouts between the in vitro models. The measured and modelled cell-associated concentrations of the toxicants over time in each in vitro assay are subsequently used to parameterise a human physiologically-based pharmacokinetic (PBPK) model to derive patient specific biologically equivalent doses.
CEFIC LRI ECO36 project
The aim of the CEFIC LRI ECO36 project, entitled ‘Paving the Way for QIVIVE – From nominal to free to cellular concentrations in in vitro assays’, is to develop and optimise methodologies to measure free and cellular concentrations of toxicants in vitro in order to validate the prediction models of these concentrations.
CAAT Resolving the Black Box project
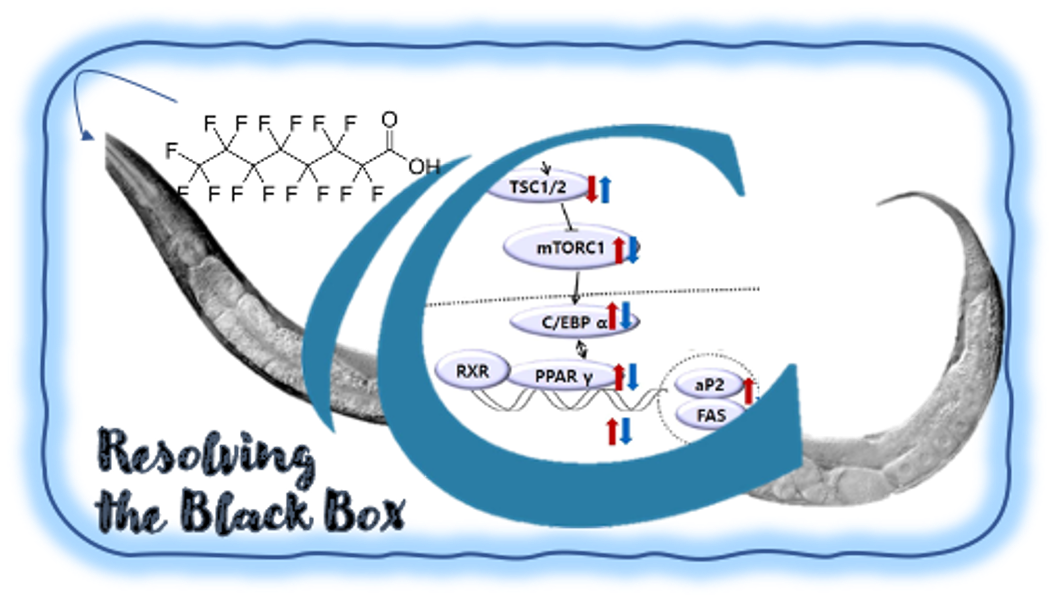 The aim of the project is to use the nematode C. elegans and toxicokinetics-toxicodynamics (TK-TD) modelling to quantify adverse outcome pathways (AOP) perturbed by perfluorinated alkylated substances (PFAS).
The aim of the project is to use the nematode C. elegans and toxicokinetics-toxicodynamics (TK-TD) modelling to quantify adverse outcome pathways (AOP) perturbed by perfluorinated alkylated substances (PFAS).
Regulatory toxicity testing is moving towards faster, mechanistically informed 3R (Replacement, Reduction and Refinement of animal testing) approaches. In vitro cell-based assays form an integral part of these new approaches, but lack the ability to quantitatively link whole-body adverse outcomes with human-relevant molecular events that have been perturbed by chemicals at a cellular level. To overcome this hurdle “Resolving the black box” proposes to integrate a whole organism 3R approach into an in vitro toxicity testing approach using the nematode Caenorhabditis elegans. This project makes use of advances in molecular biology, computational modelling and analytical chemistry, which are not routinely used in regulatory toxicity testing. The proposed interdisciplinary, multi-tiered approach incorporates TK-TD modelling to build and quantify AOPs, the progression of toxic events across scales of biological organisation, in the nematode. Developmental, reproductive and neurotoxicity AOPs perturbed by perfluorinated alkylated substances (PFAS) will be used as a proof-of-principle as (1) testing for these toxicological outcomes currently require large numbers of vertebrate animals, (2) the AOPs are complex and involve multiple cell types, and (3) the TK and TD of PFAS chemicals are poorly understood, despite their widespread occurrence in the environment. The project is led by Dr. Hughes at the HAN Biocentre and is funded by the Centre of Alternatives to Animal Testing (CAAT).
3Rs-Incorporation of the BBB in rat and human PBPK models

The aim of the 3R projects is to, as a proof-of-principle, show the importance of refining physiologically-based pharmacokinetic models by implementing a blood-brain barrier.
To determine brain concentrations and neurotoxic potencies of chemicals during drug development and chemical safety assessment, currently rats are the golden standard, despite known species differences. According to the OECD test guidelines, 25-50 rats should be used to determine the kinetics and neurotoxicity of one single compound over time. These tests are costly and raise ethical and biological concerns as the kinetics of a chemical in the human body and thus neurotoxic potencies differ significantly from animals because, amongst others, the blood-brain barrier (BBB) permeability differs.
Therefore, we will construct rat and human physiologically-based pharmacokinetic (PBPK) models with and without a BBB to serve as a proof-of-principle of this approach to replace animal use in neurotoxicity testing for humans in the future. Using in vitro BBB models, the uptake of neurotoxicants over the BBB can be determined for rats and humans. Also, in vitro functional toxicity assays will be done to assess neurotoxic concentrations in the rat brain, which serve as input into the PBPK model to simulate (differences in) doses in rats and humans leading to neurotoxicity.
This project will work towards reducing the uncertainty associated with species differences, making the implementation of PBPK models as a replacement for animal studies in regulatory toxicology feasible in the near future. The project is funded by the Animal Welfare Body Utrecht.

